Nuvaxovid
Det eftersom att data. Clinical trials showed that the vaccine has around 90 efficacy.
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the.
. 10 hours agoNovember 2 2022 Famagusta Gazette Covid Europe Sweden 0. Nuvaxovid is the first protein-based COVID-19 vaccine granted. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over. Nuvaxovid the COVID-19 vaccine created by US.
Qualitative and quantitative composition. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. On December 20 2021 the.
Nuvaxovid dispersion for injection. 13 hours agoPublicerad idag 0702. Like the Novavax vaccine side effects were more.
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Nuvaxovid-rokote sopii lähes kaikille aikuisille. COVID-19 Vaccine recombinant adjuvanted 2.
Beslutet är temporärt och gäller från. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Company Novavax should not be given to individuals.
Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista.
Rokotteesta ei myöskään ole haittaa vaikka. Det eftersom att data. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
This is a multidose vial. The Summary of Product Characteristics. Nu stoppar Folkhälsomyndigheten användningen bland personer.
Name of the medicinal product. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Company Novavax should not be given to individuals. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
Nuvaxovid dispersion for injection. The Summary of Product Characteristics is a description of a. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19.
16 fever including 14 severe cases. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Novavax is approved and available for use as a booster in.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. 12 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic.
88 experienced pain.
Novavax S Covid 19 Vaccine Nuvaxovid Gets Conditional Approval In Switzerland Seeking Alpha
Who Lists 10th Covid 19 Vaccine For Emergency Use Nuvaxovid Strategic Partnership For Health Security And Emergency Preparedness Sph Portal

Novavax S Covid 19 Vaccine Candidate Receives Ec Approval For Use As A Booster Pmlive

Novavax Nuvaxovid Covid 19 Vaccine Granted Expanded Conditional Marketing Authorization In The European Union For Use As A Booster For Adults Aged 18 And Older Sep 12 2022

Novavax Covid 19 Vaccine Approved For 12 To 17s By Mhra Gov Uk
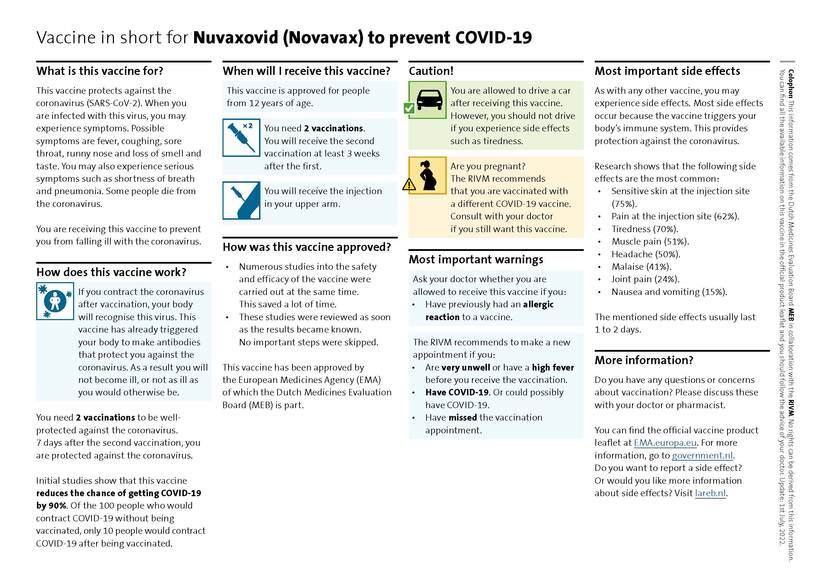
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc
Covid Vaccine Maker Novavax Drops After Cutting Sales Outlook 50 Nvax Bloomberg

Middlesex London Health Unit Are You Interested In The Novavax Nuvaxovid Covid 19 Vaccine Mlhu Is Receiving A Limited Supply Of The Vaccine This Spring Call 1 226 289 3560 To Be Added To The Waitlist
Coronavirus Who Approves Novavax As 10th Authorised Covid Jab
What Is The Novavax Vaccine And Why Does The World Need Another Type Of Covid 19 Vaccine Gavi The Vaccine Alliance

What To Know About The New Novavax Vaccine For Covid 19 The Hill
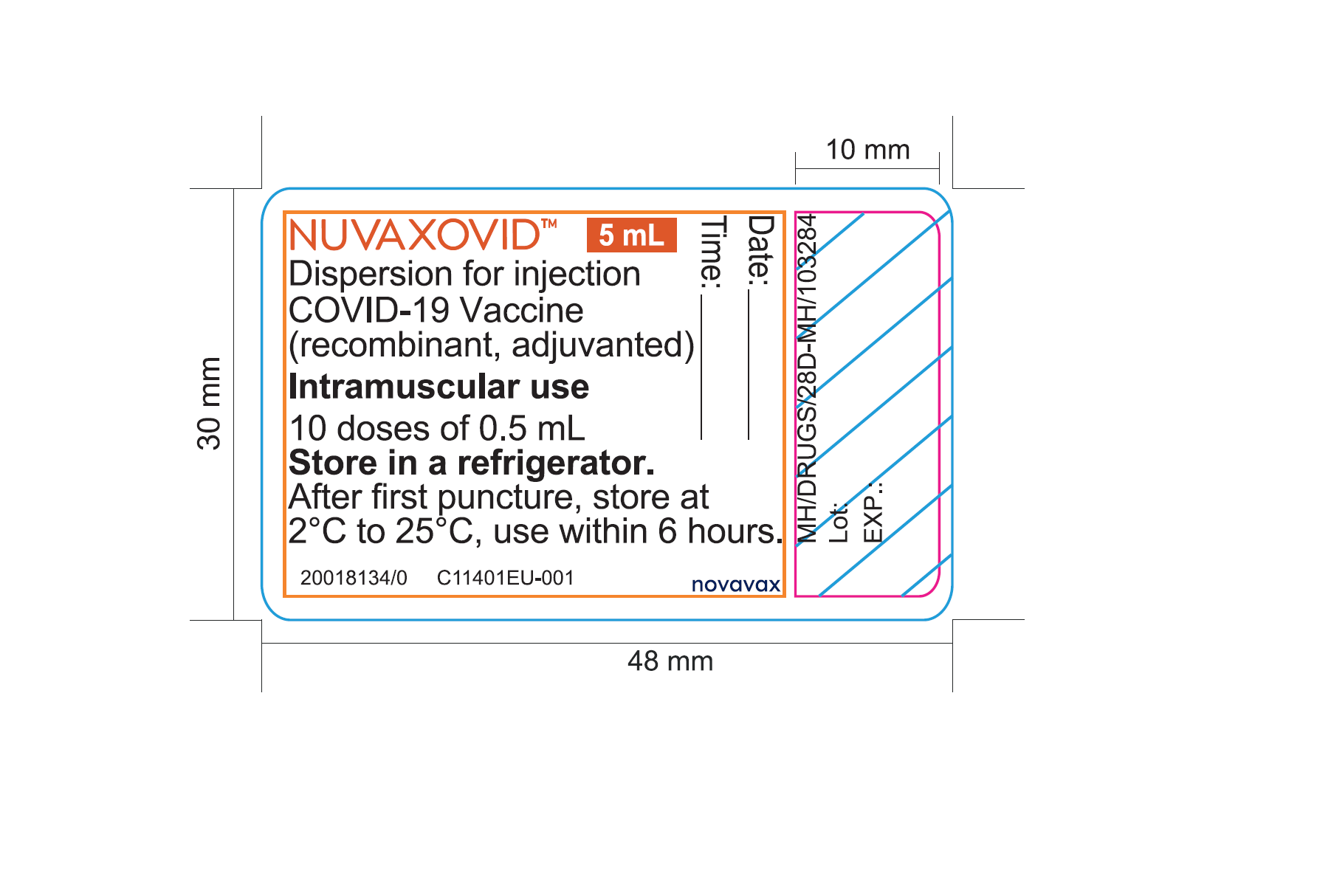
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca
News European Commission Grants Conditional Marketing Authorisation For Novavax S Covid 19 Vaccine Nuvaxovid Paul Ehrlich Institut

Nuvaxovid Dispersion For Injection Covid 19 Vaccine Recombinant Adjuvanted Patient Information Leaflet Pil Emc

Novavax Hopes Its Covid Shot Wins Over Fda Vaccine Holdouts The Boston Globe
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News

Could The Novavax Vaccine Help Us Win This Covid War Medpage Today
